Reducing Underuse and Overuse in Healthcare
Enabling Better Medical Decisions
Generating and Disseminating Evidence
About Eviva Partners
Over the past decades, medical research has produced tremendous advances in diagnosis, prevention, treatment, and follow-up of disease, resulting in the development of many evidence-based practices. Yet their use has remained low for decades or has been declining (the underuse gap). In contrast, the use of low-value, ineffective or harmful practices is increasing (the overuse gap). Eviva Partners was founded to address these care gaps by improving medical decision-making.
Our foundational hypothesis is that some patients and providers experience unmet evidence needs (UENs)—areas where existing evidence may be extensive but not easily accessible, or where specific questions have not yet been studied directly.
We recognise that for many interventions, including vaccines, large bodies of evidence already demonstrate strong safety and effectiveness; however, specific concerns sometimes arise around particular sub-questions that warrant clearer communication or targeted investigation. Through both evidence synthesis and, where appropriate, new research, Eviva aims to bridge these gaps to support better, evidence-informed decisions.
We envision a world in which all patients have access to adequate and clearly communicated evidence to enable the best medical decisions.
Our guiding principles are as follows:
Bringing together experts from diverse disciplines – public health, epidemiology, clinical research, communication science, marketing, and others.
Defining our goals and tracking progress through research, evaluation, and measurement.
Two-eyed seeing: respect, bidirectional trust, and common values. No politics. No blame.
Honest communication, acknowledging uncertainty where it truly exists, while also conveying where the science is already strong and settled.
Our Approach
-
Our qualitative research suggests that the vast majority of vaccine-hesitant individuals in the US appear to demonstrate science-consistent reasoning, asking rational questions about, for example, the safety of vaccine ingredients and the vaccine schedule.
These individuals are not getting satisfactory answers to these questions from providers or the medical establishment, in part because the evidence to support such answers does not exist. We therefore refer to their concerns as unmet evidence needs (UEN).
3. As a consequence, instead of the details they seek, or frank discussions of knowns and unknowns, the patients hear generic statements such as “vaccines are safe and effective,” which they do not accept.
4. Worse, they describe being dismissed and ridiculed for asking questions. As a result, they feel ostracized and isolated.
-
Eviva’s approach is to distinguish between:
Areas where strong evidence already exists but has not been effectively conveyed, and
Areas where a genuine evidence gap remains, meriting further study (e.g., aluminum adjuvants and asthma).
Our goal is to map these public unmet evidence needs and address them through a dual approach:
Science communication—clarifying and contextualising existing evidence.
Targeted evidence generation—commissioning new studies only where biologically plausible and methodologically feasible.
We refer to this integrated approach as “EFP,” or Evidence For the People.
-
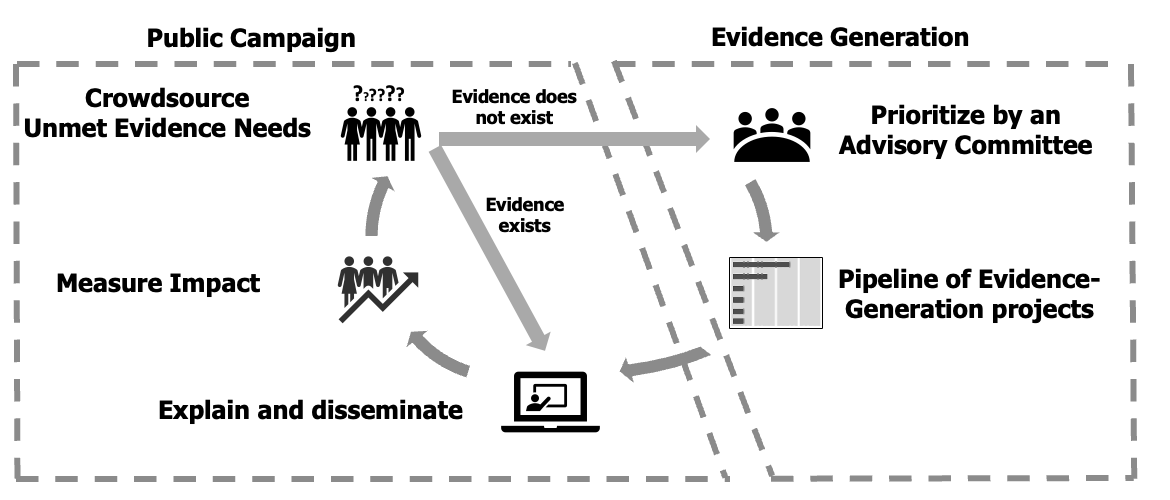
Eviva begins by mapping or “crowdsourcing” Unmet Evidence Needs among the public and healthcare providers.
Where strong evidence already exists, it will be synthesised and disseminated through communication campaigns that make the science accessible and relatable.
Where evidence truly does not exist or remains uncertain, new studies will be commissioned using rigorous, transparent methods.Eviva will be firewalled from the design, conduct, analysis, and reporting of these projects to preserve independence, while ensuring adherence to research best practices:
i. Pre-registration and timely publication of all results.
ii. Oversight by qualified, independent experts.
iii. Inclusion of diverse study populations, with stratified analyses where possible.Both existing and newly generated evidence will be disseminated, and their impact on public understanding and vaccination coverage will be measured.
Eviva Team & Advisory Board
Alex Morozov
CEO, Founder
Alex Morozov is a physician-scientist, drug developer and a writer with a passion for improving health outcomes. He spent about 15 years in the pharmaceutical industry overseeing hundreds of clinical trials of new cancer treatments. While writing a book about medical evidence, he met Elder Marshall who inspired him to start Eviva.
-
Alex obtained his undergraduate degree from MIT, followed by combined MD-PhD training at Albert Einstein College of Medicine in New York. After completing internal medicine residency at Columbia-Presbyterian and medical oncology fellowship at Memorial Sloan-Kettering Cancer Center in New York, Alex was recruited by a friend to join the pharmaceutical industry. Over his 15-year drug development career, Alex has designed, conducted and overseen hundreds of clinical trials, leading to regulatory approval of several new cancer therapies by the FDA and other health authorities around the world. Following his interest in data and digital innovation, he led teams developing and implementing new technologies to accelerate clinical trials, address health inequity, and improve outcomes. In 2024, Alex left pharma to start Eviva.
Daniel Salmon
Johns Hopkins University
Dr. Salmon is the Director of Institute for Vaccine Safety at Johns Hopkins University. His primary research and practice interest is post-licensure vaccine safety, developing systems and science in vaccine safety, and developing and evaluating provider, practice and patient interventions to improve vaccine informed decision-making.
Ashley Anderson
University of North Carolina
Ashley Anderson is Professor of Political Science at the University of North Carolina, Chapel Hill. A specialist in comparative politics, her research interests include contentious politics, authoritarian regimes, and vaccine hesitancy in minority populations.
-
Dr Anderson is working on a book on variation in union responses to political movements in authoritarian regimes. In addition to this work, newer research projects examine the dynamics of opposition movements in authoritarian regimes more broadly, with applications to state-mobilized contention and Islamist party success
-
Dr. Salmon is broadly trained in vaccinology, with an emphasis in epidemiology, behavioral epidemiology, and health policy. He has considerable experience developing surveillance systems, using surveillance data for epidemiological studies, and measuring immunization coverage through a variety of approaches. Dr. Salmon has worked with state, federal and global public health authorities to strengthen immunization programs and pandemic planning. Note: Participation by Dr Salmon does not constitute or imply endorsement by the Johns Hopkins University or the Johns Hopkins Hospital and Health System.
Julie Williams
Executive Director
Julie Williams brings over 12 years of experience from Abt Global where she specialized in evaluating large-scale social programs. Julie has a robust background in designing and implementing evaluations and is proficient in quantitative and qualitative research methodologies, data collection and management, and analysis techniques.
-
Julie’s expertise extends to managing multi-million-dollar projects, client relations, report writing, and dissemination of findings. She holds a Master of Public Policy from Georgetown University. When she’s not working, you’ll find Julie browsing the shelves of the local bookstore, taking her youngest to the park, or cheering on her eldest’s softball team.
Michael Holmes
High School of American Studies
Michael Holmes teaches science at The High School of American Studies at Lehman College, a specialized high school in New York City and one of the top public schools in the US. He obtained a Master’s degree based on his research at the Environmental Protection Agency, Albert Einstein College of Medicine and the Population Council.
-
Bridging the gap between applied science and classroom learning has lead Mr. Holmes in garnering numerous awards. He is the recipient of NYC Teaching Fellowship, Sloan Award for Excellence in Teaching Science and Mathematics by the Fund for the City of New York, An Astor Fellowship at NYU and a runner-up for the Mueller Award in teaching sponsored by Math For America. He served as the lead chemistry teacher in the College Now program at Bronx Community College.
Dietram Scheufele
University of Wisconsin, Madison
Dr. Dietram Scheufele is Professor and Taylor-Bascom Science Communication Chair at the University of Wisconsin. He is one of the leading voices globally developing effective strategies for communicating with and engaging different audiences, particularly marginalized ones, with science.
-
Elder Marshall is from the Moose Clan of the Mi’kmaw Nation; he lives in the community of Eskasoni in Unama’ki – Cape Breton, Nova Scotia. He is the recipient of multiple Honorary Doctorates (Cape Breton University, Acadia University, Humber College) and numerous other awards, including the Order of Canada (2023).
Gordon Guyatt
McMaster University
Dr. Gordon Guyatt is a Distinguished Professor in the Department of Health Evidence and Impact at McMaster University. He coined the term “evidence-based medicine” (EBM) in 1991, and since then has been a leading advocate of evidence-based approaches to clinical decision-making. He is one of the world’s most-cited living scientists.
-
Dr Guyatt was the lead editor for the “Users’ Guides to the Medical Literature,” a guide aimed at helping clinicians integrate the principles of evidence-based medicine into their clinical practice. He also played a key role as the lead methodologist in the creation of 19 COVID-19 guidelines, which were developed in partnership with the World Health Organization. “GRADE” refers to a systematic approach to rating the quality of evidence and grading strength of recommendation that Dr Guyatt played a key role in developing and is now used by over 110 organizations worldwide including the WHO, the Cochrane Collaboration, and UpToDate.
Helen Petousis-Harris
University of Auckland, NZ
Dr Petousis-Harris is a Vaccinologist, Co-Director of the Global Vaccine data Network, Director of the Vaccine Datalink and Research Group (VADAR), and Associate Professor, in the School of Population Health at Waipapa Taumata Rau, University of Auckland. Her main research areas are vaccine safety and vaccine real-world evidence.
-
Dr Petousis-Harris trained in biological sciences and molecular medicine prior to moving into vaccines and vaccination. She has been involved in immunisation-related research in New Zealand since 1998, encompassing clinical, social science, epidemiological, and health systems research. Her main research areas are vaccine safety and vaccine real-world evidence. She is a previous Chair of the World Health Organization Global Advisory Committee on Vaccine Safety (GACVS). She serves as a media spokesperson on vaccines and vaccination, as well as a science communicator.
Elder Albert Marshall
Mi’kmaw Nation
Dr Elder Albert Marshall is the author of the “Etuaptmumk/Two-Eyed Seeing” methodology, a guiding principle for the co-learning journey of different cultural knowledges working together. This approach has been the subject of hundreds of publications and has been adopted by researchers and government agencies around the world.
-
Dr Scheufele’s current research examines how algorithmically curated information environments fundamentally reshape how we all make sense of the world around us. His most recent publications have included work on mis- and disinformation, open science, and the societal impacts of emerging technologies like AI and human brain organoids. His consulting portfolio includes work for DeepMind, Porter Novelli, PBS, WHO, and the World Bank.